Nitric Acid Lewis Structure. Draw the lewis structure for nitric acid (the hydrogen atom is attached to one of the oxygen atoms). Include all three resonance structures by alternating the double bond among the three oxygen atoms.
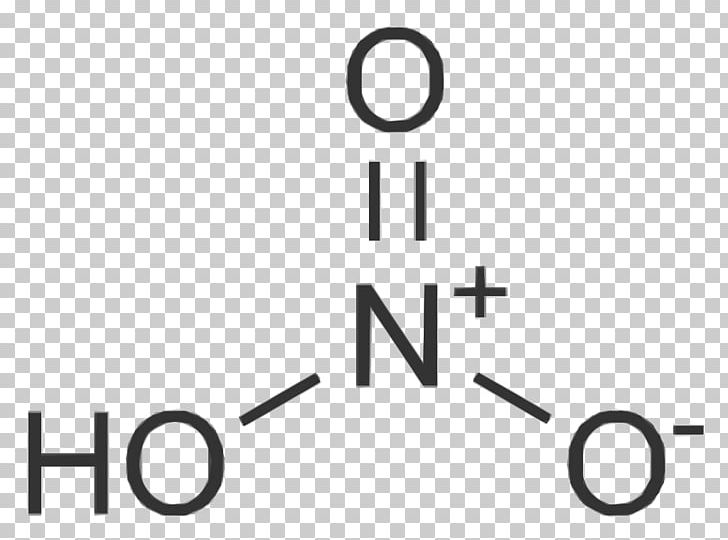
This is known as electron dot structure, another name for lewis structure. Nitric acid is an interesting customer in terms of lewis structure, given that two of the five constituent atoms bear formal charges. The chemical formula hno2 represents nitrous acid.
And Of Course One Of The Oxygen Bears A Formal Negative Charge.
In hno3 molecules, one of the oxygen atoms is doubly bonded to the central nitrogen atom. Another oxygen atom is singly bonded to the central nitrogen atom and also singly bonded to a hydrogen atom. Producing ammonium nitrates is used to manufacture plastic, dye, and fertilizer
Let Us Consider The Case Of The Lewis Electron Dot Structure Of Nitric Acid Hno 3 (Hno 3 Lewis Structure).
A lewis structure for no would look like: You know that, nitrogen (n) has 5 balance electron & oxygen (o) has 6. Nitric acid molecules contain 3 oxygen atoms, 1 nitrogen atom, and 1 hydrogen atom.
Can Be Used As Content For Research And Analysis.
O = group 6 = 6 valence electrons. There are no lone pairs on nitrogen atom and also there are charges on one oxygen atom and nitrogen atom. Total electrons = 5 + (3x6) + 1 = 24 electrons.
Nitric Oxide Is Composed Of A Single Nitrogen Atom That Is Bonded To A Nitrogen Atom.
Mg + 2hno3 → mg (no3)2 + h2 mn + 2hno3 → mn (no3)2 + h2. It is a conjugate acid of a nitrate. H = group 1 = 1 valence electron.
Hno3 Structure Or Nitric Acid Structure:
Hno 2 (nitrous acid) lewis stricture is drawn step by step by using total valence electrons of each element. Draw the lewis structure for nitric acid (the hydrogen atom is attached to one of the oxygen atoms). Here are a number of highest rated nitric oxide lewis structure pictures on internet.
Related Posts
- C6H5Nh3No2 Acid Or BaseC6H5Nh3No2 Acid Or Base. Join yahoo answers and get 100 points today. See the answer see the answer done loading.Predict whether the following salts ...
- Lewis Structure Of N2Lewis Structure Of N2. The molecular geometry of n2 is linear. The lewis structure shows the positions of each of the atoms and also shows the molecu ...
- Bromous Acid Lewis StructureBromous Acid Lewis Structure. The total number of electrons would 8. The bonding between the different atoms in covalent molecules is shown by some d ...
- No2 Dot StructureNo2 Dot Structure. No 2 is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use prima ...
- Seof2 Lewis StructureSeof2 Lewis Structure. For math, science, nutrition, history. Determine the shape, the ideal bond angle, and the direction of any deviation for seof2 ...
- Lewis Dot Structure Ch3FLewis Dot Structure Ch3F. Show the charges on the molecule above if the charge for the atom is something other than zero. Chapte 10odnom con question ...
- Lewis Dot Structure For Co32Lewis Dot Structure For Co32. This problem has been solved! Check the formal charges to make sure you have the best lewis structure.Note that we do n ...

