Seof2 Lewis Structure. For math, science, nutrition, history. Determine the shape, the ideal bond angle, and the direction of any deviation for seof2.
It has a linear molecular geometry and sp3 hybridization. That gives us a total of 14 valence electrons for the nh2cl lewis structure. Of2 has a bent shape and a tetrahedral electron geometry.
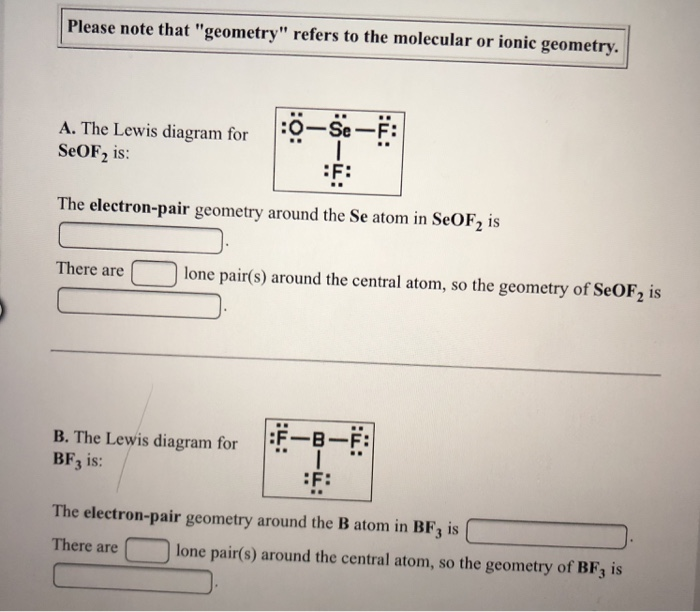
Draw The Best Lewis Structure, And Resonance Contributors Of Equal Energy (If Any), For The Molecule Seof2.
This electron arrangement is known as 'trigonal bipyramidal. A rapid explanation of seo2's molecular geometry including a description of seo2 binding angles. The actual structure of seo2 is a resonance hybrid of all three structures.
Seo 2 Is A Lewis Structure With Selenium (Se) Which Can Hold More Than 8 Valence Electrons.;
Pcl5 has a molecular weight of 208. It has a linear molecular geometry and sp3 hybridization. In all three structures, there are three electron domains about the se atom:
That Gives Us A Total Of 14 Valence Electrons For The Nh2Cl Lewis Structure.
The electron geometry around se is trigonal planar. The first structure you come up won't have formal charges equal to zero and therefore is. The two oxygen atoms are arranged adjacent to the central sulfur atom.
Answer = Seof2 Is Polar.
Determine the shape, the ideal bond angle, and the direction of any deviation for seof2. The lone pair and the bonds on either side. Quiz your students on seof2 lewis structure, molecular geometry, bond angle, polar or nonpolar using our fun classroom quiz game quizalize and personalize your teaching.
Polar Molecules Must Contain Polar Bonds Due To A Difference In Electronegativity Between The Bonded Atoms.
Seof2 has a pyramidal shape with one lone pair of electrons on the central se atom. Calculate the total number of valence electrons present. There are lone pair s around the central atom, so the molecular geometry shape of seof2 is b.
Related Posts
- Po3 Lewis StructurePo3 Lewis Structure. Question how do you write 2c2h6 in words? Po3 meaning in chemistry phosphite anion phosphite ion po4 po3 meaning trading po3 cha ...
- Draw The Lewis Dot Structure For NoDraw The Lewis Dot Structure For No. Lastly, there is a single unpaired electron on the nitrogen atom. The lewis structure for no requires you to pla ...
- Lewis Dot Structure H2SLewis Dot Structure H2S. Lewis structure of h2s has dot electron representative structure. Lewis defined a base as an electron pair donor and an acid ...
- Lewis Dot Structure For Icl5Lewis Dot Structure For Icl5. Iodine pentachloride, icl5 molecular geometry & polarity. Each lone pair of electrons contains two electrons.Choose ...
- Lewis Dot Structure For So42Lewis Dot Structure For So42. Now, we are going to learn, how to draw this lewis structure. Therefore phosphorous has five valence electrons in its l ...
- Hydroperoxyl Lewis StructureHydroperoxyl Lewis Structure. Draw the lewis structure for the polyatomic hydroperoxyl ho−2 anion. Be sure to include all resonance structures that s ...
- Nh2Ch2Cooh Lewis StructureNh2Ch2Cooh Lewis Structure. Academia.edu is a platform for academics to share research papers.Bonding in the CH2O Molecule YouTube from www.youtube.c ...



