Lewis Dot Structure For Co32. This problem has been solved! Check the formal charges to make sure you have the best lewis structure.
6 x 3 = 18 charge: Check the formal charges to make sure you have the best lewis structure. The number of valence electrons of a carbon atom is four which forms four bonds.
Therefore It Is Put In The Center Of The Dot.
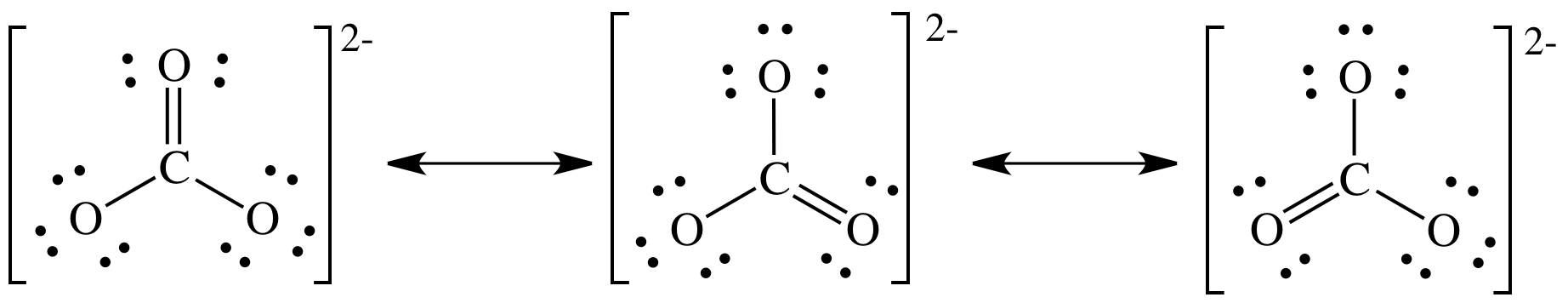
See the answer see the answer done loading. Draw the lewis structure for co32− including any valid resonance structures. Also question is, how many lewis structures does co32?
Oxygen Atoms Bears A Formal Charge Of ‐1 And All Other Atoms Are Neutral.
The correct lewis structure for this ion has one carbonoxygen double bond and two carbonoxygen single bonds. 6 x 3 = 18 charge: The lewis structure for c o32−.
4 From Carbon (Black Dots) And 3 X 6 From Oxygen (Red Dots), Making 22 Valence Electrons Plus An Extra 2 (Blue Dots) For The Additional Two Electrons That.
The carbon atom requires four electrons to obtain octet configuration whereas the oxygen atom requires two. Therefore, the valency is satisfied via the donation of a lone pair of electrons for bonding by the oxygen atom. However a third lewis structure can be drawn for so2 which is more stable in theory but doesnt quite match experimental data.
Is Co3 2 Polar Or Nonpolar?
Has one carbon‐oxygen double bond, and two carbon‐oxygen single bonds. Total valence electrons concept is. Each of the singly bonded.
(A) It Should Have The Single Bond Replaced With A Double Bond, And Two Lone Pairs On The Oxygen Atom At That End.
So, carbon has four electrons in its valence shell.oxygen is located at 6 th group. Data sheet and periodic table :0: The average of a double bond and 2 single bonds.
Related Posts
- Nh2Ch2Cooh Lewis StructureNh2Ch2Cooh Lewis Structure. Academia.edu is a platform for academics to share research papers.Bonding in the CH2O Molecule YouTube from www.youtube.c ...
- Bromous Acid Lewis StructureBromous Acid Lewis Structure. The total number of electrons would 8. The bonding between the different atoms in covalent molecules is shown by some d ...
- Bf4 Lewis Structure DotBf4 Lewis Structure Dot. Explain how to draw the lewis structure for sf2. What is the lewis structure of bf4?BrF4 Molecular Geometry YouTube from www ...
- Nitric Acid Lewis StructureNitric Acid Lewis Structure. Draw the lewis structure for nitric acid (the hydrogen atom is attached to one of the oxygen atoms). Include all three r ...
- Lewis Dot Structure For So42Lewis Dot Structure For So42. Now, we are going to learn, how to draw this lewis structure. Therefore phosphorous has five valence electrons in its l ...
- Ps2 Lewis StructurePs2 Lewis Structure. After determining how many valence electrons there are in cs2, place them around the central atom to complete the octets. Log ko ...
- Hydroperoxyl Lewis StructureHydroperoxyl Lewis Structure. Draw the lewis structure for the polyatomic hydroperoxyl ho−2 anion. Be sure to include all resonance structures that s ...




