Draw The Lewis Dot Structure For No. Lastly, there is a single unpaired electron on the nitrogen atom. The lewis structure for no requires you to place fewer than 8 valence electrons on nitrogen (n).
Determine the total number of valence electrons to be depicted in the lewis diagram. Draw the lewis dot structure for each of the following polyatomic ions: Hint lewis dot structures also known as electron dot structures.
Draw The Lewis Dot Structures For Each Of The Following Molecules:
Lewis structure for h3coh this site might help you. Hint lewis dot structures also known as electron dot structures. Draw the electron dot structure.
For The Following Molecules Or Ions (Where The Central Atom Is Underlined):
For octet purposes, oxygen has 8 electrons and nitrogen has 9. Draw the lewis dot structure for each of the following polyatomic ions: Lewis structure of nitric acid.
The Trial Structure Is You Have 14 Valence Electrons In Your Trial Structure.
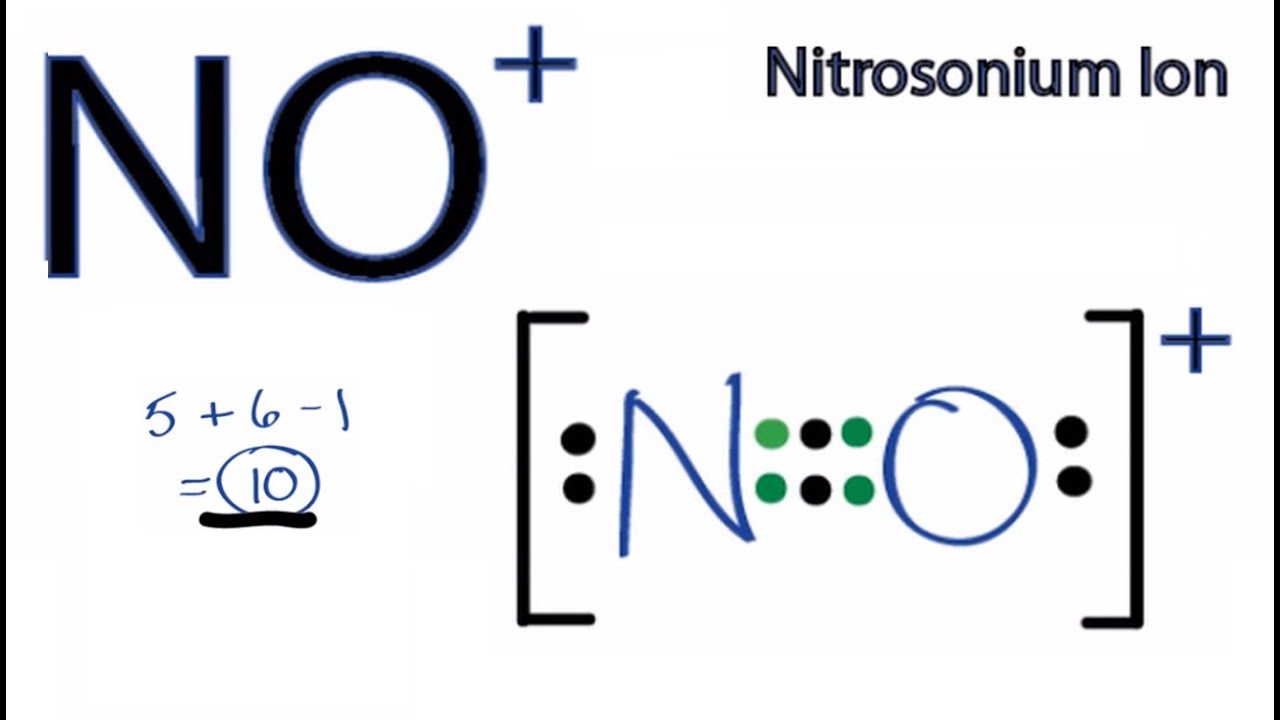
Everything you want about barium chloride lewis dot diagram will be provided by bartendery. Number of electrons in the valence shells are used to draw the stable lewis structure and it is used find the resonance structures of no. There are a total of 10 valence electrons in no +.
Lewis Dot Structures Represent The Valence Electrons Of The Atoms Present Within The Molecule.
First, draw single bonds between the atoms. (o =)n +( − o)− 2 is the typical resonance isomer. Give oxygen 2 and nitrogen 3.
The Double Bar Between The Two Chemical Symbols (=) Means That Nitrogen And Oxygen Share A Double Bond—2 Pairs Of Electrons.
Lewis, who introduced it in his 1916 article the atom and the molecule. Place least electronegative element in center and draw single bonds from the central atom to other atoms. No | nitric oxide nitric oxide is a simple molecule which contains only two atoms;
Related Posts
- Lewis Dot Structure For So42Lewis Dot Structure For So42. Now, we are going to learn, how to draw this lewis structure. Therefore phosphorous has five valence electrons in its l ...
- Seh2 LewisSeh2 Lewis. The lewis structure for h 2 se is similar to h 2 o. Sp3 then draw the 3d molecular structure using vsepr rules:Solved Please Note That &q ...
- Bf4 Lewis Structure DotBf4 Lewis Structure Dot. Explain how to draw the lewis structure for sf2. What is the lewis structure of bf4?BrF4 Molecular Geometry YouTube from www ...
- Po3 Lewis StructurePo3 Lewis Structure. Question how do you write 2c2h6 in words? Po3 meaning in chemistry phosphite anion phosphite ion po4 po3 meaning trading po3 cha ...
- Lewis Structure Of N2Lewis Structure Of N2. The molecular geometry of n2 is linear. The lewis structure shows the positions of each of the atoms and also shows the molecu ...
- Lewis Dot Structure For Icl5Lewis Dot Structure For Icl5. Iodine pentachloride, icl5 molecular geometry & polarity. Each lone pair of electrons contains two electrons.Choose ...
- Lewis Dot Structure Ch3FLewis Dot Structure Ch3F. Show the charges on the molecule above if the charge for the atom is something other than zero. Chapte 10odnom con question ...




