C6H5Nh3No2 Acid Or Base. Join yahoo answers and get 100 points today. See the answer see the answer done loading.
So we have is a. The statement which describes these determinations most. So we have the salt that dissociates into n.
See The Answer See The Answer Done Loading.
Chalk) doesn’t dissolve in water, so it doesn’t behave like a base. Let us help you simplify your studying. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine.
Dichloromethane ( Dcm Or Methylene Chloride) Is An Organochloride Compound With The Formula C H 2 Cl 2.
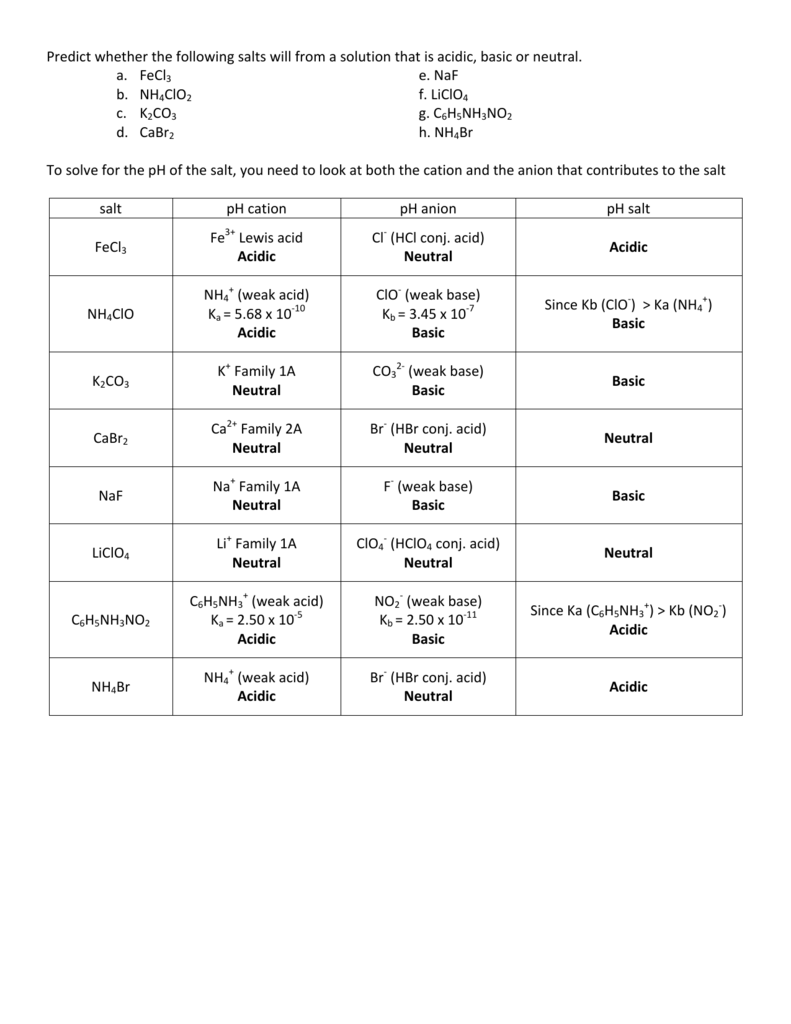
So it does not affect the nature of the salt. Potassium sulphate (k2so4) is prepared by combining koh (potassium hydroxide) and h2so4 (sulphuric acid).as h2so4 is a strong acid and koh is a strong base,so k2so4 is a neutral salt. Therefore it would be forming hno2 by accepting a proton, so the solution would be slightly acidic.
Any Cation That Came From A Weak Base Would Be Acidic.
03 miners and c two h five n h three plus. Answer to a dichloromethane solution contains a base and a neutr To determine which acid you have, you titrate a sample of the solid with naoh.
Solution X Has A Ph Of 4.35.
It’s a salt of a strong base ca(oh)2 and a weak acid h2co3. Ch3nh3cl acid or base to neutalize an acid with a base you must put the base in the acid, that will give the substance ph of 7 which is neutral. Our videos prepare you to succeed in your college classes.
So Therefore This Is An Acidic Salt For Part A.
The statement which describes these determinations most. Hydrocyanic acid (hcn) is a much weaker acid than nitrous acid (hno2). The results of this reaction do appear because hno2 is a weak acid.
Related Posts
- Conjugate Acid For Ch3Nh2Conjugate Acid For Ch3Nh2. For example methylamine in water chemical reaction: Become a study.com member to unlock this answer!amines from www.slides ...
- Nitric Acid Lewis StructureNitric Acid Lewis Structure. Draw the lewis structure for nitric acid (the hydrogen atom is attached to one of the oxygen atoms). Include all three r ...
- C6H5Nh3Cl Acid Or BaseC6H5Nh3Cl Acid Or Base. 14 mol c h 12.011 g 1.0079 g 14.007 g + 18 mol h × + 2 mol n × mol c mol h mol n + 5 mol o × b. Calculate the ph of a 0.163 m ...
- Bromous Acid Lewis StructureBromous Acid Lewis Structure. The total number of electrons would 8. The bonding between the different atoms in covalent molecules is shown by some d ...

