Lewis Dot Structure For So42. Now, we are going to learn, how to draw this lewis structure. Therefore phosphorous has five valence electrons in its last shell.
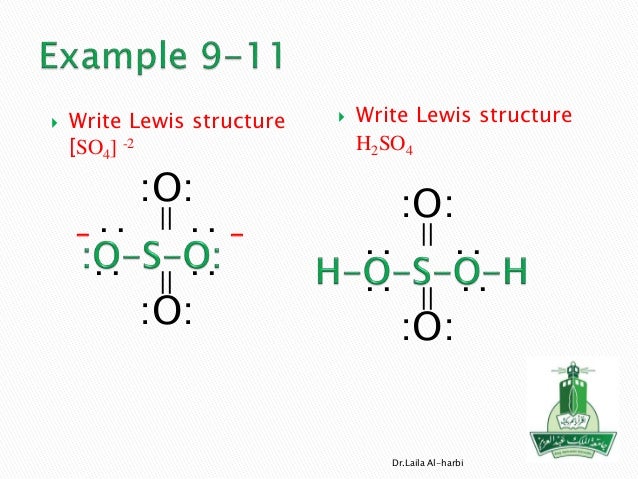
Sulfur is in group 6a so it have 6 valence electrons and oxygen has six, so fill this all in around the elements. What is the structure of so4? It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule.
The Lewis Dot Structure Of So2, Or Sulfur Dioxide, Has A Central Atom Of Sulfur That Violates The Octet Rule.
There are no lone pairs in the last shell of sulfur atom. It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. What is the difficulty of this problem?
Ttuchme1010 Teaches Viewers How To Draw The Lewis Dot Structure For Sulfate.
Drawing the lewis structure for so 2. Question 6 (4 points) resonance question: Write out the proper lewis dot structure for so42.
Draw All The Possible Resonance Structures.
Keeping this in view, what is the shape of so4 2? The given molecule is, as we know that sulfur and oxygen has '6' valence electrons. After determining how many valence electrons there are in no2 place them around the central atom to complete the octets.
Lewis Dot Structures Help Predict Molecular Geometry.
Remember, sulfur is in period 3 and can hold more than 8 valence electrons. Formal charge practice, you can also practice lewis dot structure: One is to follow the octet rule and having single bond for each oxygen each perfectly satisfies the octet rule.
The Central Atom Of Sulfur Has One Lone Pair And Is Double Bonded To Two Oxygen Atoms.
Submit the answer sheet in the exam work drop box. You might think you've got the correct lewis structure for so 4 at first. First, we will have sulfur in the middle with oxygen surrounding it.
Related Posts
- Sicl4 Lewis Structure Molecular GeometrySicl4 Lewis Structure Molecular Geometry. Is sicl4 polar or nonpolar? Welcome back to our channel, and for today’s video, we will do lewis structure ...
- Lewis Dot Structure For Co32Lewis Dot Structure For Co32. This problem has been solved! Check the formal charges to make sure you have the best lewis structure.Note that we do n ...
- Draw The Lewis Dot Structure For NoDraw The Lewis Dot Structure For No. Lastly, there is a single unpaired electron on the nitrogen atom. The lewis structure for no requires you to pla ...
- Co2 Lewis Dot StructureCo2 Lewis Dot Structure. The co2 has a linear shape, being o=c=o. A dot is placed around the atom symbol for each valence electron.38+ Lewis Structur ...
- Nh2Ch2Cooh Lewis StructureNh2Ch2Cooh Lewis Structure. Academia.edu is a platform for academics to share research papers.Bonding in the CH2O Molecule YouTube from www.youtube.c ...
- Lewis Structure Of N2Lewis Structure Of N2. The molecular geometry of n2 is linear. The lewis structure shows the positions of each of the atoms and also shows the molecu ...
- Lewis Dot Structure For Icl5Lewis Dot Structure For Icl5. Iodine pentachloride, icl5 molecular geometry & polarity. Each lone pair of electrons contains two electrons.Choose ...




