Bf4 Lewis Structure Dot. Explain how to draw the lewis structure for sf2. What is the lewis structure of bf4?
On each side there is a single bond with an f atom. Corrosive to metals and tissue. It is used in electroplating, metal cleaning and making diazo salts.
So, If You Type That Structure Into Google, You Should Receive The Lewis Structure.
The reason why a fluorine ion exists in this molecule is that normally when boron and fluorine combine, they form bf3: Because that would answer your 3 outer electron question as the minus charge means an extra electron is present which would provide the one electron each for each flourine. Each f atom then has three pair of dots, one on each unbonded side.
Manufactured From The Reaction Of Boron Oxides And Hydrogen Fluoride, The Chemical Compound Bf3 Has A Pungent Smell And Is Colorless In Nature.
Tetrafluoroboric acid is a boron fluoride. On each side there is a single bond with an f atom. Each f atom then has three pair of dots, one on each unbonded side.
Bf3 Has A Total Of 24 Valence Electrons, Which We Have To Set Around The Central Atom.
Corrosive to metals and tissue. To know about bf3 lewis structure, we have to calculate the total number of valence electrons for the bf3 molecule. On each side there is a single bond with an f atom.
Watch Our Lewis Dot Structures:
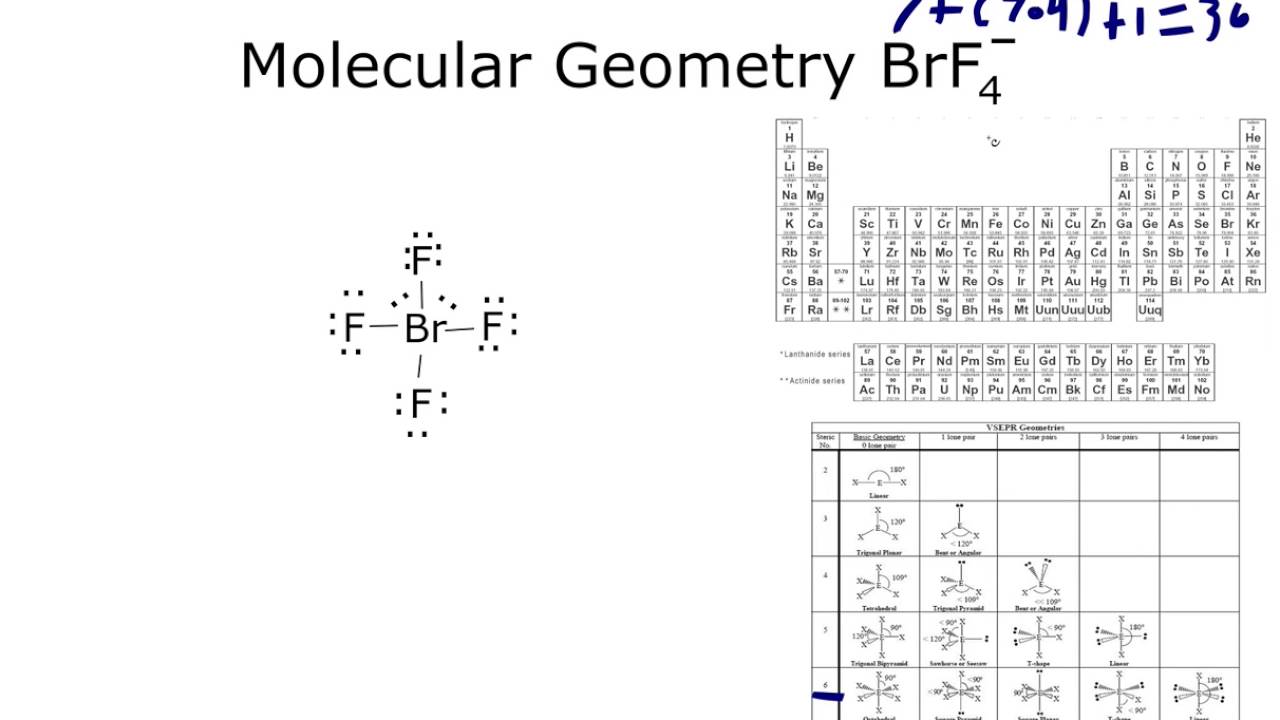
The molecular weight of this compound is calculated to be 108.6 g/mol. H2noh 2) determine formal charge of the marked atoms: The dot structure for bf4 starts with the b atom in the center.
Exceptions Video And Text Solutions To Help You Complete Your Homework.
Need to revisit the concept? The dot structure for bf4 starts with the b atom in the center. Pcl3 (phosphorus trichloride) lewis structure.
Related Posts
- Lewis Structure Ch3Nh2Lewis Structure Ch3Nh2. This is the best answer based on feedback and ratings. Identify the correct lewis structure for ch3nh2.CH3NH2 [Chemical Struc ...
- Seo2 LewisSeo2 Lewis. The two oxygen atoms are placed alongside it in the skeletal structure, as shown in the figure. Polar in chemistry, polarity is a separat ...
- Lewis Dot Structure For NafLewis Dot Structure For Naf. A dot is placed around the atom symbol for each valence electron. Click on a bond to create multiple bonds.Sodium Electr ...
- Po3 Lewis StructurePo3 Lewis Structure. Question how do you write 2c2h6 in words? Po3 meaning in chemistry phosphite anion phosphite ion po4 po3 meaning trading po3 cha ...
- Br03 Lewis StructureBr03 Lewis Structure. Identify the molecular geometry of clbr 3. It has an expanded octet (which is fine because it is above period three).NF3 Lewis ...
- Sicl4 Lewis Structure Molecular GeometrySicl4 Lewis Structure Molecular Geometry. Is sicl4 polar or nonpolar? Welcome back to our channel, and for today’s video, we will do lewis structure ...
- Lewis Dot Structure Ch3FLewis Dot Structure Ch3F. Show the charges on the molecule above if the charge for the atom is something other than zero. Chapte 10odnom con question ...



