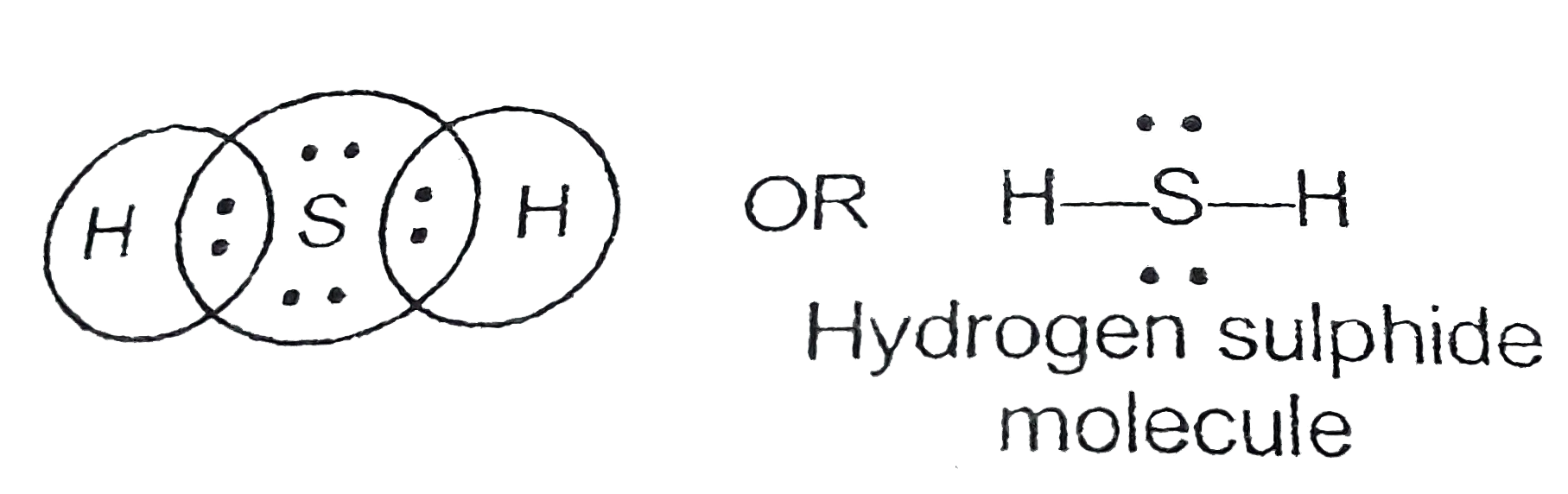
Lewis Dot Structure H2S. Lewis structure of h2s has dot electron representative structure. Lewis defined a base as an electron pair donor and an acid as an electron pair acceptor.
Total valence electrons concept is used to draw the lewis structure of h 2 so 4.sulfuric acid is a strong dibasic acid. As we have previously discussed , hydrogen only requires two electrons to fill up its valence shell because it only has a 1s shell as the very first element in the periodic table. The lewis structure of h2s is as below.
First And Foremost It Is Important To Determine How Many Valence Electrons Are Present In The Compound.
Hydrogen is in group 1 and therefore has only one valence electron. Lewis structure of sulfuric acid is drawn step by step in this tutorial. The formal charge on the calcium atom of caf2 molecule= (v.
Valence Electrons Of Atoms Undergo Orbitals Mixing In The Chemical Reactions, Gives New Types Of Molecular Species Of H2S.
Count total valence electron in h2se the first and foremost step is to determine the total number of valence. But since you have two hydrogens you need to multiply by two. Hydrogen atoms only need 2 valence electrons to have a full outer shell.
Sulfur Needs Eight Electrons To Fulfill The Requirements For Octet Rule.
It determines the number of outermost valence electrons as well as the electrons engaged in the h2s e molecule’s bond formation. The molecule has actually two hydrogen atoms and a single sulfur atom. Draw the lewis structures for the following molecules and ions:
Whether You Call Them Lewis Structures, Electron Dot Structures, Lewis Electron Dot Structures, Lewis Dot Structures, Lewis Dot Diagrams, Or Lewis Dot Formulas, It Wouldn't Matter Because The Use Is Exact.
H2s is likewise a precursor for elemental sulfur. Hydrogen has 1 valence electron, but we have two hydrogens here. Electron dot structure of ethanoic acid, h 2 s, propanone and f 2
As We Have Previously Discussed , Hydrogen Only Requires Two Electrons To Fill Up Its Valence Shell Because It Only Has A 1S Shell As The Very First Element In The Periodic Table.
The molecule is nothing but a bundle of valence electrons from the atoms. Hydrogen sulfide (h2s) lewis structure. The h2s compound is formed by various laboratory chemical reactions and comprises only two elements hydrogens and sulfur.
Related Posts
- Nh2Ch2Cooh Lewis StructureNh2Ch2Cooh Lewis Structure. Academia.edu is a platform for academics to share research papers.Bonding in the CH2O Molecule YouTube from www.youtube.c ...
- Sicl4 Lewis Structure Molecular GeometrySicl4 Lewis Structure Molecular Geometry. Is sicl4 polar or nonpolar? Welcome back to our channel, and for today’s video, we will do lewis structure ...
- Lewis Dot Structure For Icl5Lewis Dot Structure For Icl5. Iodine pentachloride, icl5 molecular geometry & polarity. Each lone pair of electrons contains two electrons.Choose ...
- Lewis Dot Structure Ch3FLewis Dot Structure Ch3F. Show the charges on the molecule above if the charge for the atom is something other than zero. Chapte 10odnom con question ...
- Mno4 Lewis StructureMno4 Lewis Structure. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. On the reactant side, what is the oxidation s ...
- Bf4 Lewis Structure DotBf4 Lewis Structure Dot. Explain how to draw the lewis structure for sf2. What is the lewis structure of bf4?BrF4 Molecular Geometry YouTube from www ...
- Po3 Lewis StructurePo3 Lewis Structure. Question how do you write 2c2h6 in words? Po3 meaning in chemistry phosphite anion phosphite ion po4 po3 meaning trading po3 cha ...




