Electronic Configuration Of Vanadium. Why is the electron configuration of vanadium? Electron configuration vanadium valence level chart electronic electrons table atom.
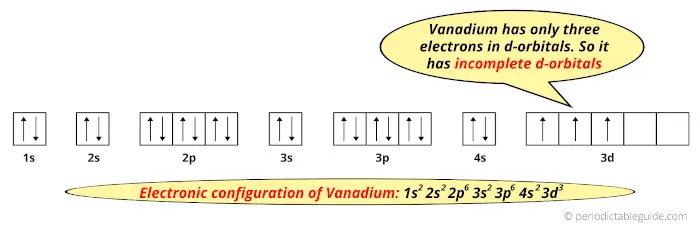
When we talk about the ground state electronic configuration firstly, it is important to know the electronic configuration of the element. Atomic spectrum representation of the atomic spectrum of vanadies. The configuration of vanadium is 22s2 22p6 3s2 4s2 3d3, which has the atomic number of 23.
The Configuration Of Argon Is 1 S 2 2 S 2 2 P 6 3 S 2 3 P 6, So Out Of The 23 Electrons, 18 Electrons Have Already Been Placed In Orbitals In A Stable Manner.
Electron configuration the periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties. What is the outermost electronic configuration of the vanadium atomic number 23 in class 11 standard? 1s2 2s2 2p6 3s2 3p6 3d3 4s2.
The General Configuration For Vanadium Will Be, V=1S22S22P63S23P64S23D3.
The creation of an oxide layer steadies the free metal against any further oxidation. 1s 2 2s 2 2p 2: However, it turns out that the 3d104s1 configuration is more stable, because that way the 3d subshell is full, which is a far more stable arrangement than 3d9.
Why Is The Electron Configuration Of Vanadium?
When we talk about the ground state electronic configuration firstly, it is important to know the electronic configuration of the element. 1s 2 2s 2 2p 3: The electronic configuration of a vanadium atom is 1s2 2s2 2p6 3s2 3p6 4s2 3d3.
1S2 2S2 2P6 3S2 3P6 4S2 3D3.
So the only possible stable configuration would be 4s2 3d3. But 4s orbital will be half filled. Due to extra stability of half filled and fulfilled orbital, cu have configuration 1s22s22p63s23p63d104s14p0.
Hence, The Expected Electron Configuration For Chromium Will Be 1S22S 2 2P 6 3S 2 3P 4 4S 2 3D 9.
An orbital diagram, like those shown above, is a visual way to reconstruct the. Titanium ← vanadium → chromium. Electron configuration of vanadium is [ar] 3d3 4s2.
Related Posts
- Electron Configuration For PhosphorusElectron Configuration For Phosphorus. Electron configuration of beryllium (be) [he] 2s 2: The electron configuration of phosphorus is #[ne] 3s^2 3p^ ...
- Which Type Of Electronic Exchange Connect Buyers With Sellers Within A Specific IndustryWhich Type Of Electronic Exchange Connect Buyers With Sellers Within A Specific Industry. 1 🔴 on a question which type of electronic exchange connec ...
- What Is The Electron Configuration Of GermaniumWhat Is The Electron Configuration Of Germanium. The electron configuration is the distribution of electrons of an atom or molecule (or other physica ...
- Electron Configuration BattleshipElectron Configuration Battleship. In milton bradley's version of the game, players secretly arrange plastic ship pieces on a 10 x 10 grid. You ...
- Cobalt Electron Configuration FullCobalt Electron Configuration Full. The electron configuration for cobalt at ground state would simply be co: Electron configuration and oxidation st ...
- Electron Configuration Of S2Electron Configuration Of S2. Note that when writing the electron configuration for an atom like fe, the 3d is usually written before the 4s. To figu ...
- Electron Configuration Of F IonElectron Configuration Of F Ion. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Dt = number of ...



