Cobalt Electron Configuration Full. The electron configuration for cobalt at ground state would simply be co: Electron configuration and oxidation states of cobalt.
What is the difficulty of this problem. What is the electron configuration of cobalt There are 2, 8, 15, 2 elements present in the 4 orbits of cobalt and can be represented in this form:
1S 2 2S 2 2P 6 3S 2 3P 6 4S 2 3D 10 4P 6 5S 2 4D 10 5P 6 6S 1 5.
The electron configuration for cobalt at ground state would simply be co: We first need to find the number of. The electron configuration for cobalt at ground state would simply be co:
The Letter Represents The Type Of Shell In Which The Electrons Sit, While The Final Number Denotes The Number Of.
The element cobalt can be found in the 4th row or 4th energy level of the periodic table. Let us help you simplify your studying. 4) write the full electron configuration for the transition metal, cobalt, ni.
We Need To Write The Electron Configuration For The Giving Audience, And Then We Have To Write It.
It is the conjugate base of the hydrogen carbonate bicarbonate ion hco 3 which is the conjugate base of h. See the answer see the answer see the answer done. The 4s orbital is filled click to see full answer subsequently, one may also ask, how many orbitals are in cobalt?
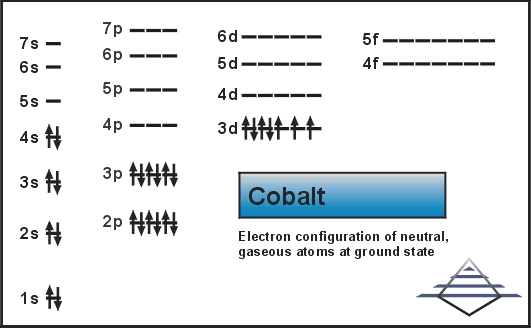
1S2 2S2 2P6 3S2 3P6 3D7 4S2.
The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the table. The oxidation state of the element changes depending on the bond formation. This list of electron configurations of elements contains all the elements in increasing order of atomic number.
5) Write The Full Electron Configuration For The Ni2* Ion.
In this case, the valence electrons of cobalt are nine. Cobalt is an inner transition metal which means the electron configuration will end in a d block. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7 3.
Related Posts
- Electronic Configuration Of BromineElectronic Configuration Of Bromine. Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the ato ...
- Electron Configuration BoronElectron Configuration Boron. 13 and it’s atomic no. Electron configuration of hydrogen (h) 1s 1:See the Electron Configuration Diagrams for Atoms of ...
- Electron Configuration Of F IonElectron Configuration Of F Ion. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Dt = number of ...
- Electron Configuration BattleshipElectron Configuration Battleship. In milton bradley's version of the game, players secretly arrange plastic ship pieces on a 10 x 10 grid. You ...
- What Is The Electron Configuration Of GermaniumWhat Is The Electron Configuration Of Germanium. The electron configuration is the distribution of electrons of an atom or molecule (or other physica ...
- Ch4 Electron Dot DiagramCh4 Electron Dot Diagram. Some possible way to show the structure of ch4 are its electron dot diagram or structural formula. Calculate the total vale ...
- Electron Configuration For PhosphorusElectron Configuration For Phosphorus. Electron configuration of beryllium (be) [he] 2s 2: The electron configuration of phosphorus is #[ne] 3s^2 3p^ ...



