Electronic Configuration Of Bromine. Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. You can see that 3p is coming before the 4p and after the 4s.
What is the name of the element group that ends with configuration ns2? The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the table. Symbol of the ground state term.
(2) (E) A Sample Of Xenon Has A R = 131.31.
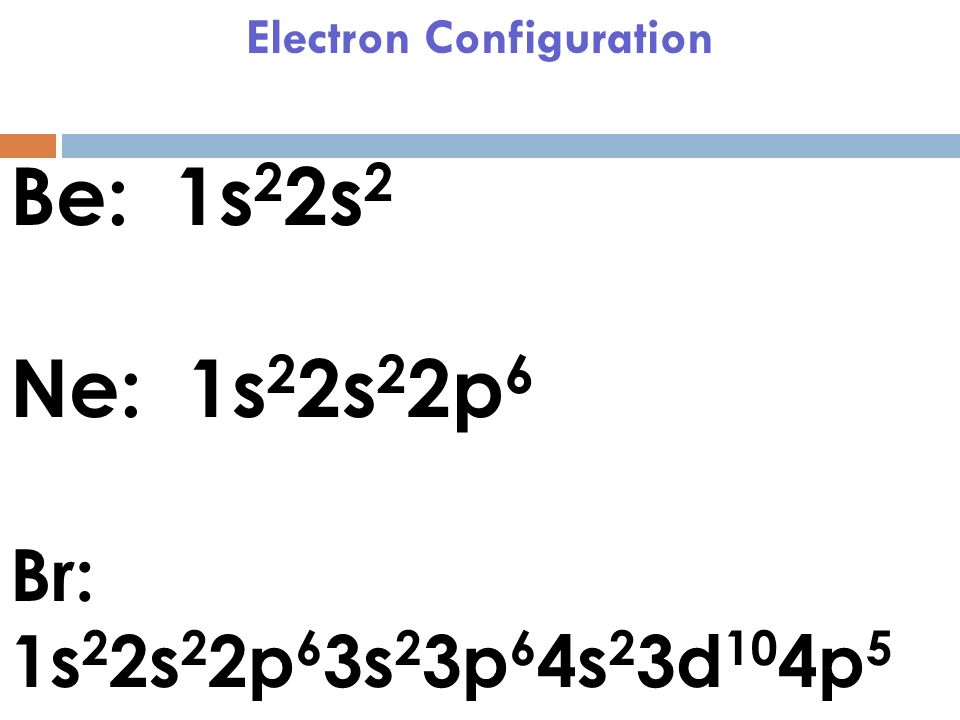
Full electron configuration of bromine: The electron holding capacity of each orbit is 2n 2. The electron configuration indicates that bromine has a.
Characteristic Electronic Configuration Of Halogens:
All halogens contain seven electrons in their outermost shell. Electron configuration chart of all elements is mentioned in the table below. 1s22s22p63s23p64s23d104p5 or [ar]4s23d104p5 answer verified by toppr upvote (0) was this answer helpful?
November 1, 2021 March 7, 2021 By Admin.
Thus, the bromine configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. Symbol of the ground state term. The electron configuration shows the distribution of electrons into subshells.
At Gcse We Are Told That Electron Configuration Is In Rings Of 2,8,8( And After That 18,18), But If This Was True Than The Structure Of Bromine Would Be 2,8,8,17?
Br (bromine) is an element with position number 35 in the periodic table. For hydrogen, it is 1s1. For example, n = 1 for k orbit.
[Ar] 4S² 3D¹⁰ 4P⁵ Is The Electronic Configuration Of Bromine.
Ar) by getting one electron. Answer well, for bromine, z = 35 and we use the aufbau principle. The electron configuration of bromine and the orbital diagram is the main topic in this article….
Related Posts
- Electron Configuration BoronElectron Configuration Boron. 13 and it’s atomic no. Electron configuration of hydrogen (h) 1s 1:See the Electron Configuration Diagrams for Atoms of ...
- Electron Configuration BattleshipElectron Configuration Battleship. In milton bradley's version of the game, players secretly arrange plastic ship pieces on a 10 x 10 grid. You ...
- Cobalt Electron Configuration FullCobalt Electron Configuration Full. The electron configuration for cobalt at ground state would simply be co: Electron configuration and oxidation st ...
- Electron Configuration For Zinc IonElectron Configuration For Zinc Ion. Which shows the electron configuration for zinc zn )? Write the complete electron configuration for the zinc ion ...
- Which Type Of Electronic Exchange Connect Buyers With Sellers Within A Specific IndustryWhich Type Of Electronic Exchange Connect Buyers With Sellers Within A Specific Industry. 1 🔴 on a question which type of electronic exchange connec ...
- Electron Configuration SilverElectron Configuration Silver. This distinctive electron configuration, with a single electron in the highest occupied s subshell over a filled d sub ...
- What Is The Electron Configuration Of GermaniumWhat Is The Electron Configuration Of Germanium. The electron configuration is the distribution of electrons of an atom or molecule (or other physica ...

