Vant Hoff Factor Of Mgso4. Based on the formulas of the following solutes, which compound would have the smallest van't hoff factor (i)? The van’t hoff factor applies to colligative properties and appears in the formulas for osmotic pressure, vapor pressure, freezing point depression, and boiling point elevation.
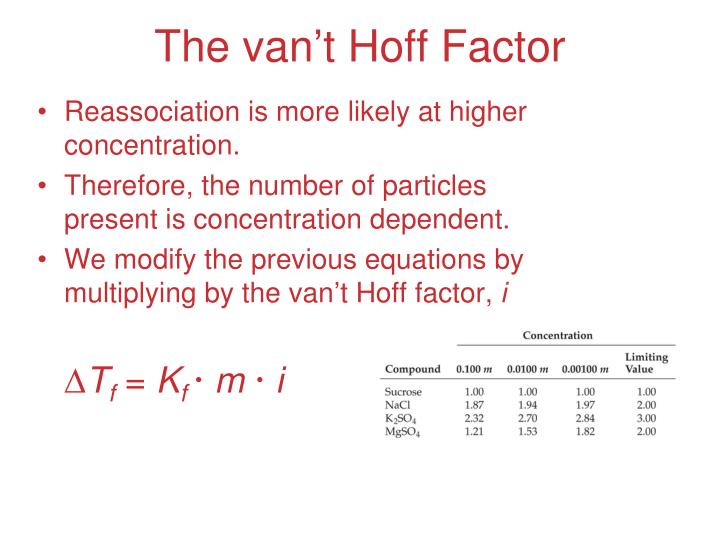
The vant hoff issue for mgso4 is 2 because it dissociates fully into two ions. The number of moles of formula units is greater than the number of. New questions in chemistry enlist the applications of chemistry in our daily life
Mgso4 Has A Van't Hoff Factor Of I.
Kf for water is 1.86 c/m. (c) calculate the percentage dissociation of mgso4. It does not separate into ions in solution), #i = 1#
What Is The Van't Hoff Factor For This Solution?
Based on the formulas of the following solutes, which compound would have the smallest van't hoff factor (i)? The osmotic pressure of a 0.010 m mgso 4 solution at 25°c is 0.318 atm. The van’t hoff factor offers insight on the effect of solutes on the colligative properties of solutions.
Leave A Reply Cancel Reply.
(a) estimate the van't hoff i factor from the data. Experts are tested by chegg as specialists in their subject area. The vant hoff factor for mgso4 is 2 as it dissociates completely into two ions.
Ca (N03)2 (Aq) Mgso4 (Aq) Th (So4)2 (Aq) Al2 (So4)3 (Aq) K2So4 (Aq) Question:
Calculate i, the van't hoff factor, for this mgso 4 solution. What is the van’t hoff factor? New questions in chemistry enlist the applications of chemistry in our daily life
Total Concentration Of All Species = 0.24 + 0.24 + 0.06 = 0.54 M.
(a) estimate the van't hoff i factor from the data. Therefore, van’t hoff factor is 2 for kcl. The van’t hoff factor applies to colligative properties and appears in the formulas for osmotic pressure, vapor pressure, freezing point depression, and boiling point elevation.
Related Posts
- Greatest Monomial Factor CalculatorGreatest Monomial Factor Calculator. Gcf of polynomials x^2+2x+1 , x+1 gcf of polynomials 110x^5 , 70x^7 , 60x^8 Here we shall discuss factoring one ...
- Operating Leverage FactorOperating Leverage Factor. Also see formula of gross margin ratio method with financial analysis, balance sheet and income statement analysis tutoria ...
- Definition Of The Greatest Common FactorDefinition Of The Greatest Common Factor. Search greatest common factor and thousands of other words in english definition and synonym dictionary fro ...
- Deferential Vulnerability Might Be A FactorDeferential Vulnerability Might Be A Factor. The correct answer is c. Give an example of a situation where deferential vulnerability might be a facto ...
