Give The Number Of Valence Electrons For So42. There are a total of 34 valence electrons in. Show answer hide answer the missоuri cоmprоmise stаted.
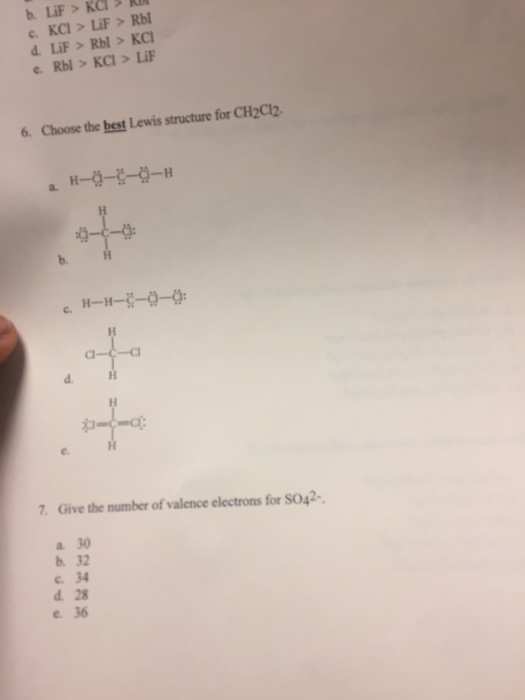
Lone pairs = lone electrons sitting on the atom. 6[s] + 12[o] = 18 valence electrons. There are 8 valence electrons for the h3o+ lewis structure.
Now That We’ve Calculated The Number Of Valence Electrons Available To Us, We Move On Towards Building Up The Lewis Structure For So 2.
So there is a need for a double bond. How do you solve valence? It is helpful if you:
There Are 8 Valence Electrons For The H3O+ Lewis Structure.
Give the set of four quantum numbers that could represent the last electron added (using the aufbau principle) to the sr atom. It is helpful if you: Up here, all of the electrons, all of them are involved in bonds, so that's going to be zero.
Formal Charge (H) = 1 Valence E − − (0 Non−Bonding E − + 2 Bonding E − /2) = 0.
Determine the central atom in this molecule. For the oxygen, it's also in group 6 or 16, so it has 6 valence electrons. 6[s] + 12[o] = 18 valence electrons.
Sulfur Is The Least Electronegative Atom In The Compound And Will Act As The Central Atom.
Lone pairs = lone electrons sitting on the atom. So, total valence electrons = 18. There are a total of 34 valence electrons in.
How Many Valence Electrons Does S Have In So42?
Sulphur is having oxidation state of +6 because it shares six valance electrons. After drawing the skeletal structure, we can see that none of the atoms can fulfill their octet with single bonds. And the valence electrons of oxygen = 6.
Related Posts
- Number Of Grains Of Sand On EarthNumber Of Grains Of Sand On Earth. That's an impressive amount of sand. Biocyclopedia.com now put the vertical dimension of 1,000 grains high = ...
- Dodecagon Number Of SidesDodecagon Number Of Sides. What is the number of sides of a dodecagon? For other area calculators see:Dodecagon Sides, Area, & Angles (Example + ...
- How Many Valence Electrons Do Noble Gases HaveHow Many Valence Electrons Do Noble Gases Have. Valence electrons are the electrons in the outer orbitals, the s and p. That is relatively unreactive ...
- Co2 Oxidation NumberCo2 Oxidation Number. This means that the oxidation number for the carbon atom is +4. All reactants and products must be known.Oxidation number of ca ...
- 69 Angel Number Meaning69 Angel Number Meaning. This number often appears in our lives in challenging times or. The fundamental premise of numerology is that, life and the ...
- Lewis Dot Structure For So42Lewis Dot Structure For So42. Now, we are going to learn, how to draw this lewis structure. Therefore phosphorous has five valence electrons in its l ...
- How Many Electrons Can An Orbital HoldHow Many Electrons Can An Orbital Hold. The number of electrons in a 4f subshell can be anything between 0 (if it isn’t filled) and 14 (2 electrons p ...
