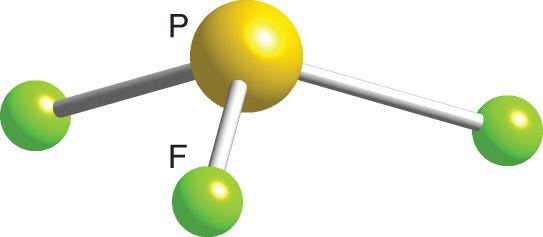
Phosphorus Trifluoride Shape. The molecule of phosphorus trifluoride (with trigonal pyramidal molecular geometry) is tilted, the bond angles between phosphorus and fluorine are 97 degrees. You can also check an article related to the polarity of pf3.
It has a difference in electronegativity values between phosphorus and The phosphorus trifluoride (pf3) molecule is classified as a polar molecule. Based on vsepr theory, polar molecules like pf3 are polar due to the lone pair that creates repulsion.
Lewis Structure Of Phosphorus Trifluoride (Pf3) The Lewis Structure Is Drawn Using Eight Dots Of Valence Electrons Around The Symbols Of The Atom With Lines Showing Bond Formation.
This polar chemical is a gas that is very poisonous [1]. Phosphorus trifluoride hydrolyses especially at high ph, but it is less hydrolytically sensitive than phosphorus trichloride. The table shows element percentages for pf 3 (phosphorus trifluoride).
A) Linear B) Bent C) Trigonal Planar D) Trigonal Pyramidal E) Tetrahedral
The lone pair of electrons repels the electrons in the three covalent bonds formed between phosphorus and fluorine to give the molecule a trigonal pyramidal shape, where the fluorine atoms form the base of the pyramid. The molecule of phosphorus trifluoride (with trigonal pyramidal molecular geometry) is tilted, the bond angles between phosphorus and fluorine are 97 degrees. The geometry of ph3 is pyramidal.
Isotope Pattern For Pf 3.
Shape of ph3 is trigonal pyramidal. Phosphorus and fluorine together form phosphorus trifluoride, or pf3. You can also check an article related to the polarity of pf3.
One Electron Group Means One Lone Pair, One Single Bond One Double Bond, Or One Triple Bond What Phrase Best Describes The Arrangement Of These Electron Groups Around The Central
Based on vsepr theory, polar molecules like pf3 are polar due to the lone pair that creates repulsion. Dig into the news of phosphorus trifluoride shape. Answer the questions in the table below about the shape of the phosphorus trifluoride (pf3) molecule.
According To Vsepr Theory, Polar Molecules Are Due To The Lone Pairings That Generate Repulsion.
Also know, what is the molecular geometry of phosphorus trifluoride? What does pf3 look like? Phosphorus trifluoride (formula p f 3), is a colorless and odorless gas.it is highly toxic and reacts slowly with water.
Related Posts
- Molecular Shape Of Bef2Molecular Shape Of Bef2. Be has a 1s22s2 electron configuration with is no unpaired. Property name property value reference;Bef2 Molecular Orbital Di ...
- Molecular Shape For Ch4Molecular Shape For Ch4. The molecule has quite a symmetry in its arrangement as there are bonds on all four sides of the central atom. The central a ...
- F Orbitals ShapeF Orbitals Shape. For l = 3 therefore, there are seven f orbitals. 2) orbitals are combined when bonds form between atoms in a molecule.What is the s ...
- Nh2Cl Molecular ShapeNh2Cl Molecular Shape. The molecular geometry of nh2cl is trigonal pyramidal with asymmetric charge distribution on the central atom. The shape of th ...
- Electron Configuration For PhosphorusElectron Configuration For Phosphorus. Electron configuration of beryllium (be) [he] 2s 2: The electron configuration of phosphorus is #[ne] 3s^2 3p^ ...
- Clo3 Shape And Bond AngleClo3 Shape And Bond Angle. According to vsepr theory, c l o 3 − ion has pyramidal geometry due to the presence of 1 lone pair and 3 σ bond pairs whic ...
- Shape Of Cocl2Shape Of Cocl2. It is readily soluble in water, alcohol, and acetone. One electron group means one lone pair, one single bond, one double bond, or on ...




