Number Of Valence Electrons In Magnesium. So, during chemical reaction, it gives up these 2 electrons in its outermost orbit. Valence electrons in silicon (si) 4:
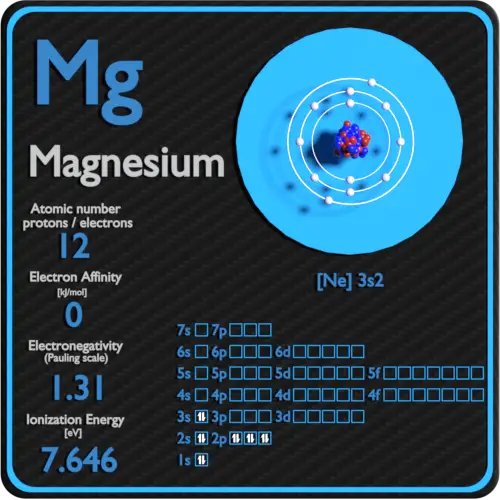
Valency of magnesium (mg) there are many different ways to find out the valency of an atom which reflects the ability of an atom to bond with other atoms. But the valency of elements, when combined with h or o first, increases from 1 to 4 and then it reduces to zero. The quantum numbers for the electrons of the valence shell are, n=3,l=0,m=0,s=+12 and n=3,l=0,m=0,s=−12.
In This Case, The Valency And Valence Electrons Of Magnesium Are 2.
Zinc has 2, 8, 18, 2, so it has 2 valence electrons. Consider two compounds containing oxygen na 2 o and f 2 o. While moving left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8.
Group Number Of Magnesium In Periodic Table:
Thus, it has 2 valence electrons. So, during chemical reaction, it gives up these 2 electrons in its outermost orbit. The electronic distribution of the valence shell of magnesium is [ne]3s2.
Find More Answers Ask Your Question Previous Next
The atomic number of magnesium is 12. This is because it can form bonds to some elements like oxygen or sulfur while also releasing hydrogen gas when exposed to water. Magnesium has twelve valance electrons, meaning it’s an atom with a metallic bonding and quite reactive.
2 Atomic Number Of Magnesium (Mg) = 12 Electronic Configuration O.
The chemical symbol for magnesium is mg. Valency of magnesium (mg) there are many different ways to find out the valency of an atom which reflects the ability of an atom to bond with other atoms. Therefore according to the pauli’s exclusion principle there at least one quantum number would be different.
Valence Electrons In Nitrogen (N) 5:
Mark me as brainliest answer plz advertisement still have questions? As, from the bohr diagram of magnesium, we got to know, it has only 2 valence electrons. The electron configuration of magnesium “ electron configuration is the distribution of electrons of an atom or molecule in atomic or molecule orbitals”
Related Posts
- Valence Electrons In CaValence Electrons In Ca. How many valence electrons does ca and sr have? For example, if we look at the ground state (electrons in the energetically ...
- Co2 Oxidation NumberCo2 Oxidation Number. This means that the oxidation number for the carbon atom is +4. All reactants and products must be known.Oxidation number of ca ...
- Double Number Line DiagramsDouble Number Line Diagrams. Use double number lines to visualize equivalent ratios and describe a ratio relationship between two quantities. Common ...
- Valence Electrons In SodiumValence Electrons In Sodium. 4 valence electrons are found in group 14 elements. Sodium, like all the group 1 alkali metals, has one valence electron ...
- The Grinch Phone Number 2019The Grinch Phone Number 2019. Then, sit back, smile and listen to the joyful sounds of a new holiday tradition in the making. What is the grinch’s ph ...
- How Many Valence Electrons Do Noble Gases HaveHow Many Valence Electrons Do Noble Gases Have. Valence electrons are the electrons in the outer orbitals, the s and p. That is relatively unreactive ...
- Number Of Valence Electrons In HalogensNumber Of Valence Electrons In Halogens. The halogens are f, cl, br, i, and at. As the number of valence electrons increases, the electronegativity v ...



