Net Cell Reaction. When the reaction is at equilibrium, there is no net change in the amount of zinc metal or copper ions in the system, so no electrons flow from the anode to the cathode. So2− 4 (aq) + 4h+(aq) + 2e− → so2(g) +2h2o(l), e∘ red = +0.20 v.
Here's how i do it. 3) net cell reaction the. To balance charge, you must add two electrons to the right hand side:
For Detailed Discussions On Different Cells Like A Galvanic Cell Or Voltaic Cell, Or About The Gibbs Energy Change Spontaneity Of A Process Visit Byju’s.
If emf of voltaic cell is more than that of external source, current flows from voltaic. When the reaction is at equilibrium, there is no net change in the amount of zinc metal or copper ions in the system, so no electrons flow from the anode to the cathode. Zn + cu 2+ ⇌ zn 2+ + cu.
The Net Cell Reaction Is.
The silver reduction has a more positive e∘ red, so reduction is more spontaneous for. Determine eo for a mg2+ solution with pt Here's how i do it.
If There Is No Longer A Net Flow Of Electrons, The Cell Can No Longer Do Electrical Work.
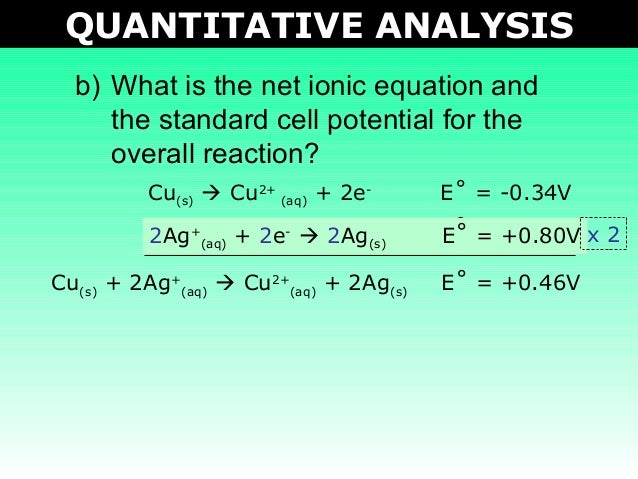
One on the anode electrode and one on the cathode electrode. The net ionic equation is a chemical equation for a reaction that lists only those species participating in the reaction. See also how much floor slope is noticeable
Click Here👆To Get An Answer To Your Question ️ Net Cell Reaction:
Find an answer to your question what is net cell reaction nishant3793 nishant3793 24.02.2020 chemistry secondary school what is net cell reaction 2 see. Oxidation zn (s) zn 2+ (aq) +. The given chemical cell follows:
The Net Cell Equation For The Given Electrochemical Cell Is Given Below.
Difference in electrical potential that arises when dissimilar metals are connected; 2 h 2 + o 2 → 2 h 2 o does not involve any ion in solution and is used in hearing aids rechargeable reaction at anode, z n → z n 2 + + 2 e − converts energy of combustion into electrical energy The net cell reaction is the sum of two electrode reactions.
Related Posts
- Predict The Major Organic Product For The Reaction BelowPredict The Major Organic Product For The Reaction Below. 8.3 18) draw the major organic product generated in the reaction below. The addition of hyd ...
- Draw The Organic Products Formed In The Following ReactionDraw The Organic Products Formed In The Following Reaction. Include the reagents, reaction conditions, observations, and chemical equations in your a ...
- Water Passes Quickly Through Cell MembranesWater Passes Quickly Through Cell Membranes. 2 get other questions on the subject: It wants to have an equal concentration on either side of the memb ...