Electron Dot Diagram Nitrogen. It is used to show how the electrons are arranged around individual atoms in a molecule. Nitrogen gas (diatomic nitrogen) watch later.
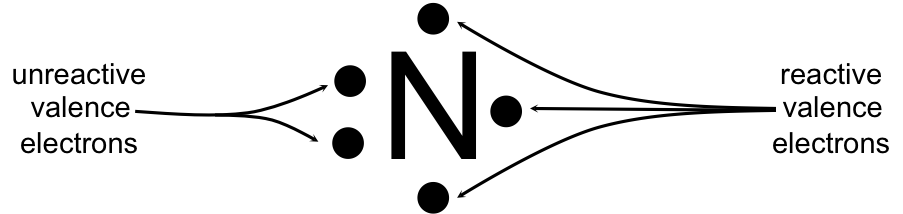
The five dot represent the five valence electrons of nitrogen. The lewis dot resonance structures of nitrogen monoxide (no) are as follows: So, nitrogen is the central atom that has 1 lone pair and 3 bonded pair electrons according to the nf3 lewis dot structure.
Hydrogen Atom Has 1 Valence Electron Hydrogen Atom Needs One More Electron To Complete Its Valence Shell, That Is, To Make 2 Electrons In Its K Shell.
Which lewis diagram represents an atom in the ground state for a group 13 element? It then is single bonded to three hydrogen atoms and has one lone pair. How easy to make electron dot diagrams.
How To Draw The Lewis Dot Structure For N2:
Where n in this case is 2 since no consists of two atoms. If you are talking about the lewis dot diagram then n 2 would have 5 dots around each of the letter n's, so that there would be 6 dots total what is the lewis dot structure for n2 look like? Electron dot diagram also called lewis structure which represents the valence electrons of atoms.
The Atomic Number Of Nitrogen Is 7 And Its Symbol Is ‘N’.
See what the community says and unlock a badge. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. This system represents an atom and its valence electrons.
Hence The Formula Of Nf3 Becomes Ax 3 N 1 So, According To The Vsepr Chart , If The Molecule Has The Formula Of Ax 3 N 1 , It Has A Molecular Shape Of Trigonal Pyramid And Electron Geometry Of Tetrahedral.
Dot lewis diagram nitrogen valence electron atoms electrons lithium molecules covalent atom bohr bond biology primer lab unpaired amp figure. Nitrogen goes in the centre. No2 dot octet lewis structure electron odd rule nitrogen dioxide bond chemistry electronic molecule.
Find The Component Form Of The Velocity Of The Airplane, Assuming Tha.
Lewis dot diagrams, atoms and ions 1. Nitrogen atom (group 15) has 5 valence electrons nitrogen atom needs 3 more electrons in order to complete its valence shell, that is, to make up 8 electrons in the l shell. Nitrogen gas (diatomic nitrogen) watch later.
Related Posts
- What Is The Electron Configuration Of GermaniumWhat Is The Electron Configuration Of Germanium. The electron configuration is the distribution of electrons of an atom or molecule (or other physica ...
- Electron Domain Of Pcl3Electron Domain Of Pcl3. The shape of pcl3 is a trigonal pyramid. What is the electronic and.PCl3 Lewis Structure and Molecular Geometry YouTube from ...
- Electron Configuration BoronElectron Configuration Boron. 13 and it’s atomic no. Electron configuration of hydrogen (h) 1s 1:See the Electron Configuration Diagrams for Atoms of ...
- The Third Wish Plot DiagramThe Third Wish Plot Diagram. Don't blame me if you spend the. The rags to riches story of maggie malone, an aspiring author who finds a rare edi ...
- Electron Configuration BattleshipElectron Configuration Battleship. In milton bradley's version of the game, players secretly arrange plastic ship pieces on a 10 x 10 grid. You ...
- Electron Configuration For Zinc IonElectron Configuration For Zinc Ion. Which shows the electron configuration for zinc zn )? Write the complete electron configuration for the zinc ion ...
- Electron Configuration SilverElectron Configuration Silver. This distinctive electron configuration, with a single electron in the highest occupied s subshell over a filled d sub ...

