Which Bond Is Stronger Covalent Or Ionic. Covalent bond is not as strong as ionic bond. A given nonmetal atom can form a single, double, or triple bond with another nonmetal.
Collected from the entire web and summarized to include only the most important parts of it. Sugar does not form ions in solution 8. Covalent bonds are stronger if you compare with ionic molecules, because their molecular orbital overlap is bigger.
Some Ionic Bonds Are Stronger And Some Covalent Bonds Are Stronger.
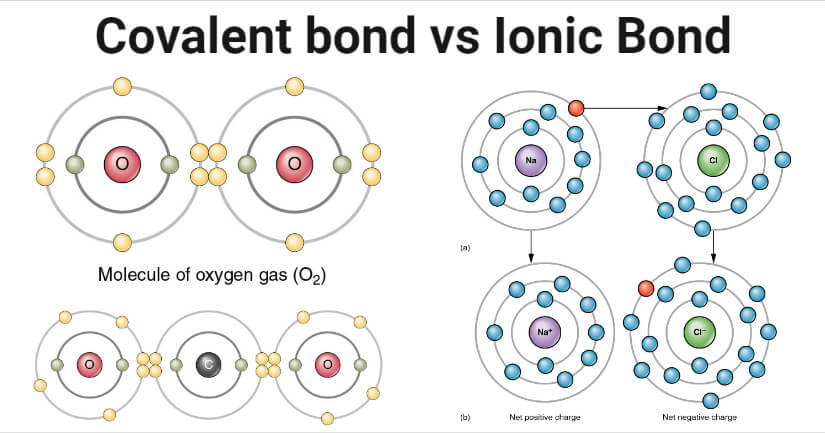
Covalent bonding a covalent bond, also referred to as molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms.these electron pairs and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is what is referred to as covalent bonding. How are ionic and covalent bonds formed? Ionic bonds are stronger than covalent bonds, because there is a stronger attraction between ions that have opposite charges, which is why it takes a lot of energy to separate them.
Sugar Does Not Form Ions In Solution 8.
Ionic bonds are stronger than covalent bonds, because there is a stronger attraction between ions that have opposite charges, which is why it takes a lot of energy to separate them. Ionic bond is much stronger than covalent bond because it involves complete transfer of electrons because of which there is formation of cation and anion and there exist huge electrostatic forces of attraction. Which is stronger a covalent bond or an ionic bond?
Atoms Form Covalent Bonds In Order To Reach A More Stable State.
Which is stronger ionic or metallic bonds? A given nonmetal atom can form a single, double, or triple bond with another nonmetal. Ionic bonds can be observed on the solids at room temperature which have high melting points.
Click To See Full Answer Accordingly, Is Metallic Bond Stronger Than Ionic?
Ionic bonds are generally strong due to the attraction of oppositely charged ions. Generally, ionic bonds are considered the stronger bond due to the extreme electrostatic attraction between the ions. As we shall explore in this section on ionic bonding, ionic bonds result from the mutual attraction between oppositely charged ions.they tend to be stronger than covalent bonds due to the coulombic attraction between ions of opposite charges.
They Tend To Be Stronger Than Covalent Bonds Due To The Coulombic Attraction Between Ions Of.
Covalent bonds are bonds that involve the sharing of electron pairs between atoms. Click to see full answer. Ionic bonds result from the mutual attraction between oppositely charged ions while a covalent bond is a bond that results from a sharing of electrons between nuclei.
Related Posts
- Which Of These Phases Encompasses All Of The Stages Of Mitosis But No Other EventsWhich Of These Phases Encompasses All Of The Stages Of Mitosis But No Other Events. D) inadequate blood supply will retard the development of the tes ...
- Which Of The Following Best Describes The Process Of EluviationWhich Of The Following Best Describes The Process Of Eluviation. To do so, we must intensify and improve the efficiency of production on the best man ...
- Is Ch4 A Covalent BondIs Ch4 A Covalent Bond. The sharing of electrons between atoms of the same elements (for example n2, h2 and o2 etc). These shared electrons are found ...
- Which One Does Not Belong WorksheetWhich One Does Not Belong Worksheet. They click on the one that does not belong! On this worksheet it is all animals that can jump or run fast but on ...
- Which Action Can The Federal Reserve Take To Pursue A Tight Money PolicyWhich Action Can The Federal Reserve Take To Pursue A Tight Money Policy. Decrease the amount of money in the economy which industry did the intersta ...
- In The Group 3 To Group 12 Elements Which Subshell Is Filled Up Going Across The RowsIn The Group 3 To Group 12 Elements Which Subshell Is Filled Up Going Across The Rows. Part a in the group 3 to group 12 elements, which subshell is ...
- Which Of The Following Best Describes When A Variable Interval Schedule Provides ReinforcementWhich Of The Following Best Describes When A Variable Interval Schedule Provides Reinforcement. For example, a teacher might provide reinforcement on ...



