How Many Σ And Π Bonds Are In This Molecule. How many sigma and pi bonds are in the molecule pictured below? In a triple bond, there is one σ bond and two π bonds.
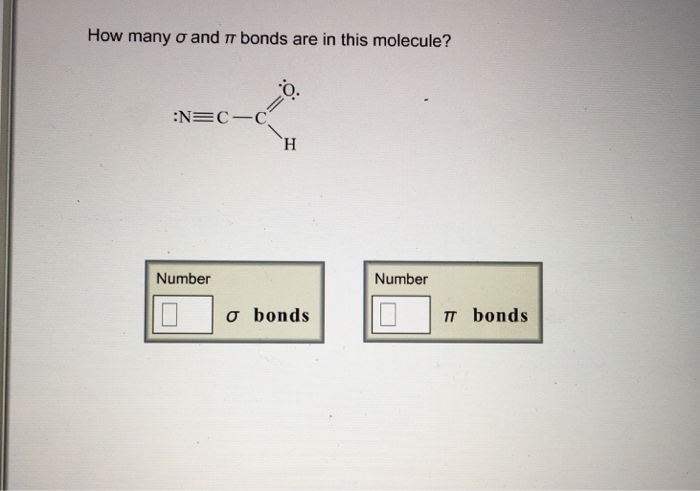
Problem 12.1 how many σ and π bonds are present in each of the following molecules? 3 📌📌📌 question how many σ and π bonds are in this molecule? How many σ and π bonds are present in a molecule of cumulene?
Every Triple Bond Contains A Sigma Bond And Two Pi Bonds.
3 📌📌📌 question how many σ and π bonds are in this molecule? What types of orbital overlap occur in cumulene? What type of hybrid orbitals are utilized by carbon in anthracene?
7 How Many Electron Pairs Are Shared Between The Carbon Atoms In A Molecule?
This plane contains the 6 atoms and all of the sigma bonds. The double bond contains 1 sigma bond and 1 pi bond and 4 hydrogen bond forms sigma bond with carbon. How many sigma and pi bonds are in the molecule pictured below?
6 Σ, 1 Π Free Expert Solution Show Answer Answer:
Thirteen sigma bonds and one pi bond b. Anthracene is a yellow, crystalline solid found in coal tar. How many σ and π bonds are in one glycine molecule?
So, Every Single Bond Is Also A Sigma Bond.
(figure 1) enter the number of σ bonds followed by the number of π bonds separated by a. A chain of five carbon atoms. Cumulene contains 7 sigma bond and 3 pi bonds.
So Ethene Contains 1 Pi Bond Between The Two Carbon Atoms And 5 Sigma Bonds In Total.
Indicate the σ and π bonds in the following molecules: Benzene molecule contains 12 σ and 3 π bonds. One σbond and one πbond.
Related Posts
- Draw All Resonance Structures For The Nitryl Chloride Molecule No2ClDraw All Resonance Structures For The Nitryl Chloride Molecule No2Cl. Draw lewis structure(s) for the nitryl chloride molecule (no2cl). Include all v ...
- How Many Feet A YardHow Many Feet A Yard. Our unit converters includes conversions for: How to convert feet to yards?How Many Cubic Feet Are In a Yard? from www.thecalcu ...
- Is Carbon Monoxide Polar Or Nonpolar MoleculeIs Carbon Monoxide Polar Or Nonpolar Molecule. Is carbon monoxide a hydrogen bond? Co2, on the other hand, is nonpolar due to its symmetrical linear ...
- 82 Kilograms Is How Many Pounds82 Kilograms Is How Many Pounds. 82.5 kg equals to 181.88 pounds or there are 181.88 pounds in 82.5 kg. One kilogram is equal 2.20462262 pounds.Which ...
- A Stick Of Butter Is How Many TablespoonsA Stick Of Butter Is How Many Tablespoons. One tablespoon of butter is equal to 1/8 of a stick or 1/2 ounce. 1 cup = 2 sticks of butter.US Sticks of ...
- How Many Ml Is 64 OzHow Many Ml Is 64 Oz. Once you know what a standard drink is you will know how much alcohol you are actually drinking. Keep reading to learn more abo ...
- How Many Ounces Are 250 GramsHow Many Ounces Are 250 Grams. To find out how many grams in ounces, multiply by the conversion factor or use the mass converter above. How many gram ...


