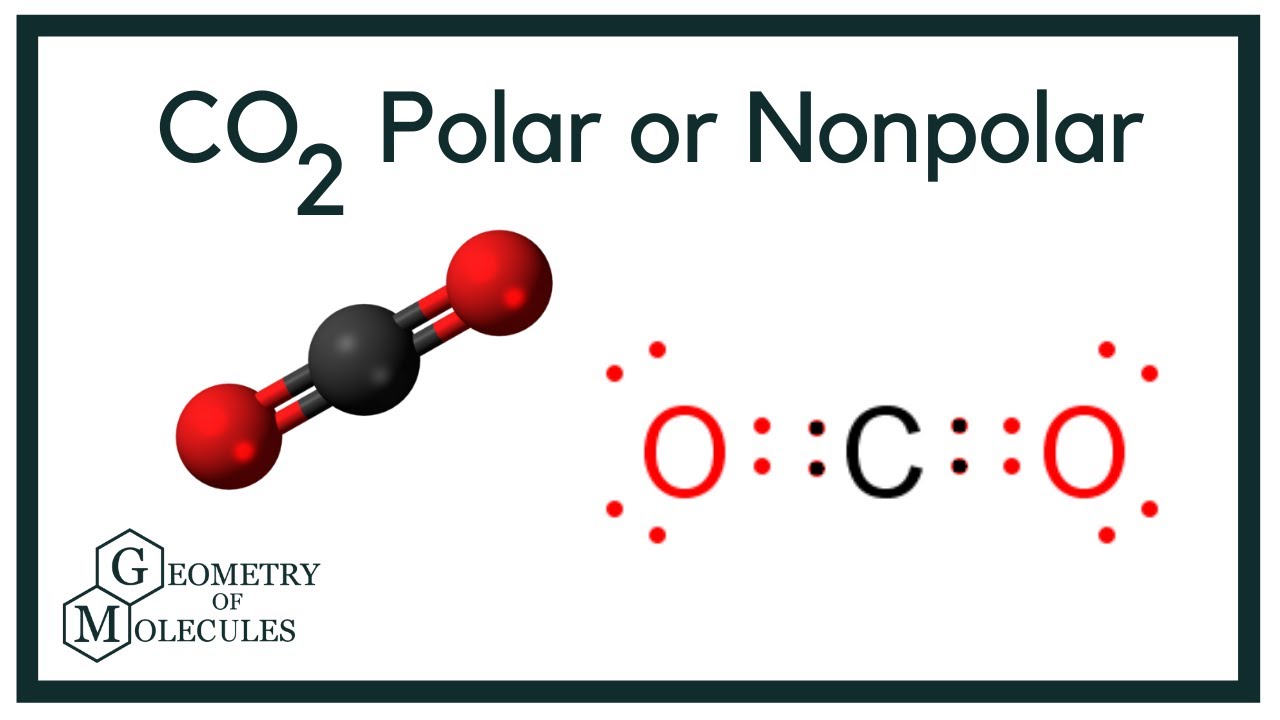
Is Carbon Monoxide Polar Or Nonpolar Molecule. Is carbon monoxide a hydrogen bond? Co2, on the other hand, is nonpolar due to its symmetrical linear structure.
Carbon monoxide is a linear molecule, but the electronegativity difference between carbon and oxygen is significant enough to make the. Other nonpolar molecules include carbon dioxide (co2) and the organic molecules methane (ch4), toluene, and gasoline. Oxygen and sulfur has same number of valance electrons in their outer most shell.
The Carbon And Oxygen Atom Have Unequal Charge Distribution And Therefore Co Bond Has A Net Dipole Moment Making Co A Polar Molecule.
Molecules can either be polar or nonpolar,. Both the atoms have lone pair of electrons and are sharing other electrons to complete their octets. Sulfur dioxide so 2, ammonia nh 3, carbon monoxide co, ethanol c 2 h 5 oh, methanol ch 3 oh, hydrogen sulfide h 2 s, chloromethane ch 3 cl, ozone o3, phosphorus trichloride (because it has trigonal pyramidal geometry) pcl 3.
Also, Is The Co Bond In Co2 Polar Or Nonpolar?
Why is carbon dioxide nonpolar and water polar? Draw the lewis structure of carbon monoxide (co) and then determine if the molecule is polar or nonpolar. Both co2 and h2o have two polar bonds.
Oxygen And Sulfur Has Same Number Of Valance Electrons In Their Outer Most Shell.
A notable exception is carbon monoxide, co. Is co2 polar or nonpolar? Pf3 is used as a ligand with metal carbonyls wherein there is a transition of metals with carbon monoxide.
A Notable Exception Is Carbon Monoxide, Co.
Co (carbon monoxide) is polar in nature because of the difference in electronegativity of carbon (2.55) and oxygen (3.44) atoms. Other nonpolar molecules include carbon dioxide (co2) and the organic molecules methane (ch4), toluene, and gasoline. It has a linear molecular geometry.
Is Carbon Monoxide A Hydrogen Bond?
In a nonpolar molecule, there are no positive or negative poles formed in the molecule. When it comes to carbon dioxide, it has a linear geometry as both the oxygen atoms share double bonds with the central carbon atom. Examples of homonuclear nonpolar molecules are oxygen (o2), nitrogen (n2), and ozone (o3).
Related Posts
- Water Is A Polar Molecule BecauseWater Is A Polar Molecule Because. A water molecule, because of its shape, is a polar molecule. The attraction between the negativeWhy Is Water a Pol ...
- Which Answer Best Describes An O3 MoleculeWhich Answer Best Describes An O3 Molecule. An ozone molecule contains one single bond and one double bond. 1.the double bond in ozone switches back ...
- How Does A Carbon Film Fossil FormHow Does A Carbon Film Fossil Form. The mud and silt form around what is left of the organism and slowly hardens and becomes sedimentary rock. A thin ...
- Draw All Resonance Structures For The Nitryl Chloride Molecule No2ClDraw All Resonance Structures For The Nitryl Chloride Molecule No2Cl. Draw lewis structure(s) for the nitryl chloride molecule (no2cl). Include all v ...
- How Many Σ And Π Bonds Are In This MoleculeHow Many Σ And Π Bonds Are In This Molecule. How many sigma and pi bonds are in the molecule pictured below? In a triple bond, there is one σ bond an ...
- Is Carbon Tetrafluoride PolarIs Carbon Tetrafluoride Polar. Carbon tetrachloride,ccl4, has a net dipole moment of zero. If we look at the bonds individually, carbon has an electr ...
- Is Kbr PolarIs Kbr Polar. A very strong type of imf between polar molecules. Nonmetals, which have a tendency to gain electrons, generally have higher electroneg ...
