Pcl3 Geometry. There are four electron domains around the central p atom in pcl3, giving it a tetrahedral electron geometry. What is the bond formation of pcl3?
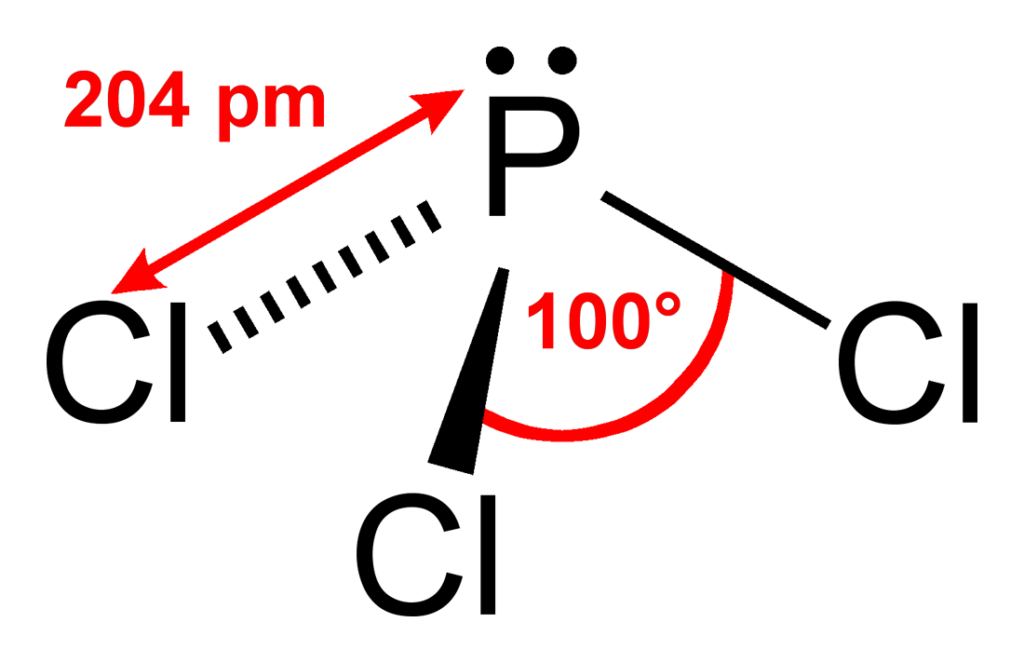
Department of health and human services. What is the bond formation of pcl3? Pcl3 what is the molecular geometry of brf5?
The Molecular Geometry Of Any Compound Can Be Determined Easily From The Vsepr Theory.
What is the geometry of xef4? Pcl3 is a polar molecule because of its tetrahedral geometrical shape having a lone pair on phosphorus atom and the difference between the electronegativity of chlorine (3.16) and phosphorus (2.19) atoms resulting in unequal sharing of electrons and develop positive and negative poles across the molecule making it a polar molecule. Square pyramidal hybridization of brf5 (bromine pentafluoride) what is the electron domain geometry around br in brf5?
Industrial Production Of Phosphorus Trichloride Is Controlled Under The Chemical Weapons Convention, Where It Is Listed In Schedule 3.
The molecular geometry, though, is trigonal pyramidal, because one of the four electron domains is a lone pair. What is the bond formation of pcl3? It undergoes sp3 hybridisation which results in tetrahedral electron pair geometry and trigonal pyramidal molecular geometry.
La Distribución Ideal De 4 Pe Es Un Tetraedro.
Pcl3 has a tetrahedral pyramidal or trigonal pyramidal molecular geometry and ammonia like electron geometry, according to the vsepr theory. In pcl3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. The shape of pcl3 molecule is a trigonal bipyramid class 11 chemistry jee_main.
B) Bipyramidal Structure Of Pcl5.
In this continuous process pcl3 is removed as it is formed in order to avoid the formation of pcl 5). Pcl3 (phosphorus trichloride) lewis structure. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98.
In Order To Achieve This, The Lone Pairs Lie In A Perpendicular Plane In An Octahedral Arrangement Opposite (180 Degree) From Each Other.
What is the lewis structure of pcl3? How many bonds are in molecule pcl3? The is mainly due to the disproportionate influence or greater repulsion of the phosphorus lone pair which makes it deviate from the ideal angle of 109 o.
Related Posts
- So2 GeometrySo2 Geometry. The molecular geometry of so2 has a bent shape which means the top has less electronegativity, and the bottom placed atoms of oxygen ha ...
- Whats Cpctc GeometryWhats Cpctc Geometry. Corresponding parts (of) congruent triangles (are) congruent if two triangles are congruent, then their corresponding parts are ...
- Electron Geometry Of Ch2OElectron Geometry Of Ch2O. Contains c h and o in the ratio ch2o? The electronegativity of oxygen is 3.44 and of carbon is 2.55 and that of hydrogen i ...
- What Is The Molecular Geometry Of Ph3What Is The Molecular Geometry Of Ph3. What is the molecular geometry of hocl? Total valence electrons pairs around phosphorous atom is four.Chapter ...
- Sicl4 Lewis Structure Molecular GeometrySicl4 Lewis Structure Molecular Geometry. Is sicl4 polar or nonpolar? Welcome back to our channel, and for today’s video, we will do lewis structure ...
- Which Statements Are True Regarding Undefinable Terms In Geometry Check All That ApplyWhich Statements Are True Regarding Undefinable Terms In Geometry Check All That Apply. Which statements are true regarding undefinable terms in geom ...
- Electron Domain Of Pcl3Electron Domain Of Pcl3. The shape of pcl3 is a trigonal pyramid. What is the electronic and.PCl3 Lewis Structure and Molecular Geometry YouTube from ...


