Arrange The Following Elements By Increasing Electronegativity. How do you arrange elements in order of increasing metallic character. C < f < li < n e.
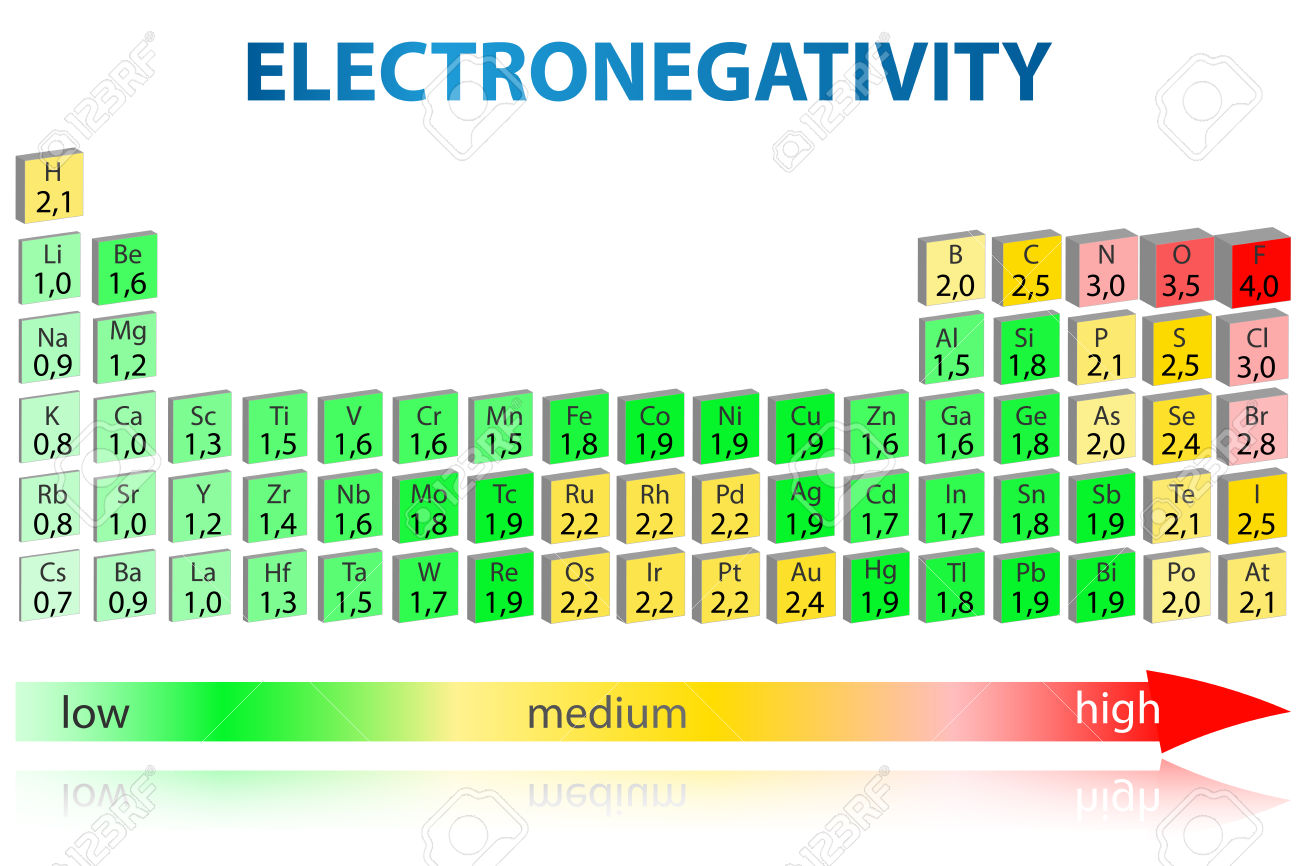
Arrange the following elements in order of increasing electronegativity: Arrange the following elements by increasing electronegativity. The energy released when an electron is added to an atom.
P, N, Si, O, Na Expert Answer 100% (71 Ratings) Order Of Increasing Electronegativity :
Thus, c < n and p < s i. Lithium , carbon , boron , beryllium please answer this question according to the general rules you have learned regarding periodic trends. Arrange the following elements in order of increasing electronegativity:
4 Rows Which List Of Elements Is Arranged In Order Of Increasing Electronegativity?
Na < si < p < n. It can also be used to predict if the resulting molecule will be polar or nonpolar. The magnitude of the negative charge on a molecule.
In A Period, As The Size Decreases, Electronegativity Increases Due To The Increase In Effective Nuclear Charge.
Electronegativity increases going across (to the right) and up the periodic table. Sulfur, oxygen, neon, aluminum neon, aluminum, sulfur, oxygen in your own words, define electronegativity and the general trend of electronegativity in the periodic table Elementsincreasing electronegativityincreasing atomic sizedecreasing electron affinity i, cl, br.
N < C < Li < F D.
I<br<cl<f going across a period fr left to right, the atomic size decrease,nuclear charge increase. na, al, p, n stackrelrarrincreasing electronegativity as we face the periodic table.electronegativity increases across a period, a row of the periodic table.and decreases down a group, a column of the periodic table. Arrange the following elements in order of increasing electronegativity (from smallest to largest) asked jun 26, 2017 in chemistry by tatiane.
Sodium (Na), Potassium (K), Nickel (Ni), Bromine (Br) K < Ni < Na < Br.
Arrange the following elements in order of increasing electronegativity: C < n < f < li c. Elementsincreasing electronegativityincreasing atomic sizedecreasing electron affinity i, cl, br.
Related Posts
- Which Of The Following Statements Are Always True About AlcoholWhich Of The Following Statements Are Always True About Alcohol. Log in for more information. Which of the following statements are always true about ...
- Which Of The Following Statements Best Describes A WikiWhich Of The Following Statements Best Describes A Wiki. Which of the following statement best describes human trafficking a. Dna is directly involve ...
- Draw The Organic Products Formed In The Following ReactionDraw The Organic Products Formed In The Following Reaction. Include the reagents, reaction conditions, observations, and chemical equations in your a ...
- Which Of The Following Best Describes The Process Of EluviationWhich Of The Following Best Describes The Process Of Eluviation. To do so, we must intensify and improve the efficiency of production on the best man ...
- In The Group 3 To Group 12 Elements Which Subshell Is Filled Up Going Across The RowsIn The Group 3 To Group 12 Elements Which Subshell Is Filled Up Going Across The Rows. Part a in the group 3 to group 12 elements, which subshell is ...
- Which Of The Following Is Not Considered A Category Of NonfictionWhich Of The Following Is Not Considered A Category Of Nonfiction. The following is a list of ongoing armed conflicts that are taking place around th ...
- During His Lifetime Christopher Marlowe Was Rumored To Be Which Of The FollowingDuring His Lifetime Christopher Marlowe Was Rumored To Be Which Of The Following. During his lifetime, christopher marlowe was rumored to be which of ...

